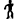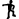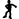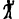Discover How This Chainmail-Inspired Material May Transform Modern Armor
Date: January 16, 2025
Did you know? You can comment on this post! Just scroll down
Source: Northwestern University
Summary: The revolutionary nanoscale material, which resembles the interconnecting links in chainmail, is extremely strong and flexible. The interlocking material has 100 trillion mechanical bonds per square centimeter, the highest density of mechanical bonds ever recorded. Small amounts of mechanically interlocked polymer added to Ultem fibers boosted the toughness of this high-performance material.
A Northwestern University-led research team has created the first two-dimensional (2D) mechanically linked material.
The nanoscale material, which resembles the interconnecting links in chainmail, is exceptionally flexible and strong. With future development, it has the potential to be used in high-performance, lightweight body armor and other applications requiring lightweight, flexible, and durable materials.
The study, published on Friday (Jan. 17) in the journal Science, represents multiple firsts in the discipline. Not only is it the first 2D mechanically interlocked polymer, but it also has 100 trillion mechanical bonds per square centimeter, the largest density of mechanical bonds ever recorded. The researchers developed this material utilizing a novel, highly efficient, and scalable polymerization procedure.
The study, published on Friday (Jan. 17) in the journal Science, represents multiple firsts in the discipline. Not only is it the first 2D mechanically interlocked polymer, but it also has 100 trillion mechanical bonds per square centimeter, the largest density of mechanical bonds ever recorded. The researchers developed this material utilizing a novel, highly efficient, and scalable polymerization procedure.
"We made a completely new polymer structure," said Northwestern's William Dichtel, the study's corresponding author. "It's similar to chainmail in that it doesn't rip easily since each of the mechanical links has some ability to move around. If you tug it, it will dissipate the force in numerous ways. And if you want to rip it apart, you'll need to break it in a variety of areas. We are still investigating its qualities and will most likely be studying it for several years."
Dichtel is the Robert L. Letsinger Professor of Chemistry at the Weinberg College of Arts and Sciences, as well as an IIN and Paula M. member. Trienens Institute of Sustainability and Energy. The study's first author is Madison Bardot, a Ph.D. candidate in Dichtel's group and an IIN Ryan Fellow.
Developing a process method
For years, researchers tried to create mechanically interlocked molecules with polymers, but it was nearly difficult to get polymers to form mechanical links.
Dichtel is the Robert L. Letsinger Professor of Chemistry at the Weinberg College of Arts and Sciences, as well as an IIN and Paula M. member. Trienens Institute of Sustainability and Energy. The study's first author is Madison Bardot, a Ph.D. candidate in Dichtel's group and an IIN Ryan Fellow.
Developing a process method
For years, researchers tried to create mechanically interlocked molecules with polymers, but it was nearly difficult to get polymers to form mechanical links.
To address this difficulty, Dichtel's team used a whole new method. They began with X-shaped monomers, the building blocks of polymers, and arranged them into a precise, highly ordered crystalline structure. They then reacted these crystals with another molecule, forming bonds between the molecules within the crystal.
"I give a lot of credit to Madison because she came up with this concept for forming the mechanically interlocked polymer," Dichtel told reporters. "It was a high-risk, high-reward idea where we had to question our assumptions about what types of reactions are possible in molecular crystals."
"I give a lot of credit to Madison because she came up with this concept for forming the mechanically interlocked polymer," Dichtel told reporters. "It was a high-risk, high-reward idea where we had to question our assumptions about what types of reactions are possible in molecular crystals."
The resulting crystals are made up of many layers of 2D interlaced polymer sheets. The ends of the X-shaped monomers are linked together within the polymer sheets. Then, additional monomers are threaded between the gaps. Despite its stiff structure, the polymer has remarkable flexibility. Dichtel's team also discovered that dissolving the polymer in solution allowed the layers of interlocked monomers to peel away from one other.
Professor Dichtel explained that the polymer's structure is relatively weak. When placed in a solvent, the crystalline part dissolves, but the individual 2D layers remain intact. This allows for the manipulation of these sheets.
To analyze the polymer's structure at the nanoscale, researchers at Cornell University, headed by Professor David Muller, employed advanced electron microscopy techniques. The resulting images demonstrated the polymer's high level of crystallinity, confirmed its interlocking structure, and highlighted its significant flexibility.
Dichtel's team also discovered that the new material can be made in vast quantities. Previous polymers with mechanical linkages were often produced in very tiny amounts using procedures that are unlikely to be scalable. Dichtel's team, on the other hand, produced half a kilogram of their novel material and believes that higher volumes are achievable when their most potential applications emerge.
Adding strength to difficult polymers.
Adding strength to difficult polymers.
Dichtel's associates at Duke University, lead by Professor Matthew Becker, added the material to Ultem after being inspired by its natural strength. Ultem, a member of the Kevlar family, is an extremely strong material capable of withstanding severe temperatures as well as acidic and caustic chemicals. The researchers created a composite material using 97.5% Ultem fiber and 2.5% 2D polymer. This modest proportion significantly boosted Ultem's overall strength and toughness.
Dichtel believes his group's novel polymer will have a future as a specialist material for lightweight body armor and ballistic materials.
Dichtel believes his group's novel polymer will have a future as a specialist material for lightweight body armor and ballistic materials.
"We have a lot more analysis to do, but we can tell that it improves the strength of these composite materials," Dichtel said. "Almost every property we have measured has been exceptional in some way."
steeped in Northwestern history.
The scientists dedicated their research to the memories of Sir Fraser Stoddart, a former Northwestern chemist who pioneered the concept of mechanical bonding in the 1980s. Finally, he transformed these linkages into molecular machines that can switch, spin, contract, and expand in controlled ways. Stoddart, who died last month, got the 2016 Nobel Prize in Chemistry for his efforts.
The scientists dedicated their research to the memories of Sir Fraser Stoddart, a former Northwestern chemist who pioneered the concept of mechanical bonding in the 1980s. Finally, he transformed these linkages into molecular machines that can switch, spin, contract, and expand in controlled ways. Stoddart, who died last month, got the 2016 Nobel Prize in Chemistry for his efforts.
"Molecules don't just thread themselves through each other on their own, so Fraser developed ingenious ways to template interlocked structures," explained Dichtel, a postdoctoral researcher in Stoddart's lab at UCLA. "However, even these approaches fall short of being practical enough for usage in large compounds such as polymers. In our current study, the molecules are firmly held in place within a crystal, which serves as a template for the creation of a mechanical bond surrounding each one.
"So, these mechanical bonds have deep tradition at Northwestern, and we are excited to explore their possibilities in ways that have not yet been possible."
"So, these mechanical bonds have deep tradition at Northwestern, and we are excited to explore their possibilities in ways that have not yet been possible."
The study, "Mechanically interlocked two-dimensional polymers," was primarily supported by the Defense Advanced Research Projects Agency (contract number HR00112320041) and Northwestern's IIN (Ryan Fellows Program).
Story Source:
Materials provided by Northwestern University. Original written by Amanda Morris. Note: Content may be edited for style and length.
Article Posted 4 Months ago. You can post your own articles and it will be published for free.
No Registration is required! But we review before publishing! Click here to get started
One Favour Please! Subscribe To Our YouTube Channel!
468k
Cook Amazing Nigerian Dishes, Follow Adorable Kitchen YouTube Channel!
1.1m
Like us on Facebook, Follow on Twitter
React and Comment
Click Here To Hide More Posts Like This
Watch and Download Free Mobile Movies, Read entertainment news and reports, Download music and Upload your own For FREE.
Submit Your Content to be published for you FREE! We thrive on user-submitted content!
But we moderate!